Artificial Intelligence
The Global Fetal Monitoring Devices Market to Register Immense Growth at CAGR of ~7% by 2027 | DelveInsight
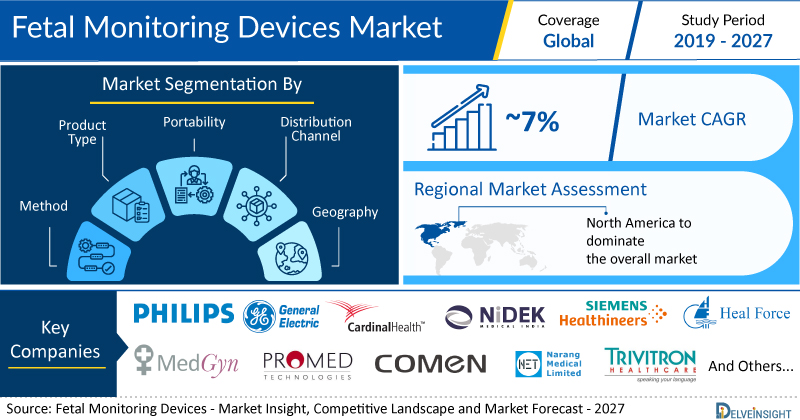
New York, USA, Jan. 17, 2023 (GLOBE NEWSWIRE) — The Global Fetal Monitoring Devices Market to Register Immense Growth at CAGR of ~7% by 2027 | DelveInsight
The global fetal monitoring device market is anticipated to grow positively owing to the increased need for monitoring fetuses for various congenital disabilities and the prevalence of preterm births and post-term pregnancy, the development of technologically advanced fetal monitors, the growing adoption of minimally invasive procedures, and the rise in government and non-government initiatives for maternal and fetal health around the world.
DelveInsight’s Fetal Monitoring Devices Market Insights report provides the current and forecast market analysis, individual leading Fetal Monitoring Devices companies’ market shares, challenges, fetal monitoring devices market drivers, barriers, and trends, and key fetal monitoring devices companies in the market.
Key Takeaways from the Fetal Monitoring Devices Market Report
- As per DelveInsight estimates, North America is anticipated to dominate the global fetal monitoring devices market during the forecast period.
- Notable fetal monitoring devices companies such as Koninklijke Philips N.V., General Electric Company, Cardinal Health, Nidek Medical, Siemens Healthcare GmbH, Heal Force, Medgyn Products, Inc., Promed Technology Co., Limited, Shenzhen Comen Medical Instruments Co., Ltd., Narang Medical Limited, Trivitron Healthcare, Neoventa Medical AB, Huntleigh Healthcare Ltd, EDAN Instruments, Inc., Shenzhen Bestman Instrument Co., Ltd., Natus Medical Incorporated, CooperSurgical Inc., Contec Medical Systems Co., Ltd, Life Plus Healthcare (P) Ltd., Bionet, and several others are currently operating in the fetal monitoring devices market.
- In March 2022, Butterfly Network, Inc., a digital health company transforming care with handheld, whole-body ultrasound, received a USD 5 million grant from the Bill and Melinda Gates Foundation to improve maternal and fetal health. With this grant, Butterfly will provide 1,000 healthcare workers in Sub-Saharan Africa with the Butterfly iQ+, the world’s only handheld, whole-body point-of-care ultrasound probe.
- In February 2022, CooperSurgical set to acquire Cook Medical’s Reproductive Health Division to expand its offerings of ob-gyn and fertility medical devices.
- In June 2021, Remote pregnancy monitoring company Nuvo Group announced the FDA supplemental 510(k) clearance to add a new uterine activity module to its INVU system. INVU is a physician-prescribed, wearable pregnancy monitoring system.
To read more about the latest highlights related to the fetal monitoring devices market, get a snapshot of the key highlights entailed in the Global Fetal Monitoring Devices Market Report
Fetal Monitoring Devices Overview
A fetal monitoring device is a medical device that includes a monitoring unit, cables, and electrodes. They are intended to measure, record, and display fetal heart rate, uterine contractions, and maternal blood pressure, as well as heart rate, before and during childbirth. It is a medical method used to check the health of an unborn baby to ensure a safe birth. Furthermore, it tracks chronic lung diseases, mental retardation, neonatal diseases, hypothermia, vision and hearing problems, and jaundice.

Fetal Monitoring Devices Market Insights
North America is expected to account for the largest share of the global fetal monitoring devices market out of all regions. Factors such as the increasing prevalence of various fetal birth defects and an increase in preterm births are expected to aid in the growth of the North American fetal monitoring devices Market. Furthermore, the country’s government is actively involved in maternal and fetal health initiatives throughout the country. According to the CDC 2022, several state-based birth defects programs currently track CHDs in newborns and young children. As a result of the rising prevalence of these defects in the country, there will be greater demand for treatments that use fetal monitoring devices, creating a favorable growth environment for the United States fetal monitoring devices market and the North American region.
To know more about why North America is leading the market growth in the fetal monitoring devices market, get a snapshot of the Fetal Monitoring Devices Market Outlook
Fetal Monitoring Devices Market Dynamics
The fetal monitoring devices market is experiencing increased product demand for a variety of reasons. The primary driver of product demand is an increased need to monitor fetuses for various birth defects. Furthermore, the prevalence of preterm births, post-term pregnancy, the development of technologically advanced fetal monitors, the growing adoption of minimally invasive procedures, and the increase in government and non-government initiatives for maternal and fetal health across the world are anticipated to bolster the fetal monitoring devices market growth.
However, the risks associated with invasive fetal monitoring and the high equipment cost may be some of the factors limiting the growth of the fetal monitoring devices market.
Additionally, the fetal monitoring devices market witnessed a COVID-19 outbreak, which significantly increased the use of fetal monitoring devices. People who had COVID-19 during their pregnancy were at an increased risk of complications affecting their pregnancy and developing babies. COVID-19, for example, increases the risk of having a preterm (before 37 weeks) or stillborn baby. This resulted in an increase in demand for fetal monitoring devices during the pandemic. Furthermore, the process of economic recovery with the easing of lockdown restrictions and the return of normalcy in the economic landscape post pandemically made the resumption of regular healthcare services, which will keep demand for these products on track in the fetal monitoring devices market.
Get a sneak peek at the fetal monitoring devices market dynamics @ Fetal Monitoring Devices Market Dynamics Analysis
| Report Metrics | Details |
| Coverage | Global |
| Study Period | 2019–2027 |
| Base Year | 2021 |
| Fetal Monitoring Devices Market CAGR | ~7% |
| Projected Fetal Monitoring Devices Market Size by 2027 | USD 4.72 Billion |
| Key Fetal Monitoring Devices Companies | Koninklijke Philips N.V., General Electric Company, Cardinal Health, Nidek Medical, Siemens Healthcare GmbH, Heal Force, Medgyn Products, Inc., Promed Technology Co., Limited, Shenzhen Comen Medical Instruments Co., Ltd., Narang Medical Limited, Trivitron Healthcare, Neoventa Medical AB, Huntleigh Healthcare Ltd, EDAN Instruments, Inc., Shenzhen Bestman Instrument Co., Ltd., Natus Medical Incorporated, CooperSurgical Inc., Contec Medical Systems Co., Ltd, Life Plus Healthcare (P) Ltd., Bionet, among others |
Fetal Monitoring Devices Market Assessment
- Fetal Monitoring Devices Market Segmentation
- Market Segmentation By Product: Ultrasound Devices (2D Ultrasound, 3D & 4D Ultrasound), Electronic Maternal/Fetal Monitors, Fetal Doppler Devices, Telemetry Devices, Others
- Market Segmentation By Method: Invasive, Non-Invasive,
- Market Segmentation By Portability: Portable, Non-Portable
- Market Segmentation By End User: Hospitals, Obstetrics & Gynecology Clinics, Others
- Market Segmentation By Geography: North America, Europe, Asia-Pacific, and Rest of World
- Porter’s Five Forces Analysis, Product Profiles, Case Studies, KOL’s Views, Analyst’s View
Which MedTech key players in the fetal monitoring devices market are set to emerge as the trendsetter explore @ Fetal Monitoring Devices Companies
Table of Contents
| 1 | Report Introduction |
| 2 | Executive summary |
| 3 | Regulatory and Patent Analysis |
| 4 | Key Factors Analysis |
| 5 | Porter’s Five Forces Analysis |
| 6 | COVID-19 Impact Analysis on Fetal Monitoring Devices Market |
| 7 | Fetal Monitoring Devices Market Layout |
| 8 | Global Company Share Analysis – Key 3-5 Companies |
| 9 | Fetal Monitoring Devices Market Company and Product Profiles |
| 10 | Project Approach |
| 11 | About DelveInsight |
Interested in knowing the fetal monitoring devices market by 2027? Click to get a snapshot of the Fetal Monitoring Devices Market Trends
Related Reports
Fetal and Neonatal Monitoring Devices Market
Fetal and Neonatal Monitoring Devices Market Insight, Competitive Landscape, and Market Forecast – 2027 report delivers an in-depth understanding of market trends, market drivers, market barriers, and key fetal and neonatal monitoring devices companies, including GE Healthcare, Medtronic, Siemens Healthcare, among others.
Beating Heart Stabilizers Market
Beating Heart Stabilizers Market Insight, Competitive Landscape, and Market Forecast – 2027 report delivers an in-depth understanding of market trends, market drivers, market barriers, and key beating heart stabilizers companies, including Medtronic, Getinge AB, Terumo Cardiovascular Systems Inc, Chase Medical, among others.
Congestive Heart Failure (CHF) Treatment Devices Market
Congestive Heart Failure (CHF) Treatment Devices Market Insight, Competitive Landscape, and Market Forecast – 2027 report delivers an in-depth understanding of market trends, market drivers, market barriers, and key congestive heart failure (CHF) treatment devices companies, including General Electric Company, Boston Scientific Corporation, Medtronic, among others.
Prosthetic Heart Valve Market Insight, Competitive Landscape, and Market Forecast – 2027 report delivers an in-depth understanding of market trends, market drivers, market barriers, and key prosthetic heart valve companies, including Medtronic, Boston Scientific Corporation, St, Jude Medical, among others.
Tissue Heart Valves Market Insight, Competitive Landscape, and Market Forecast – 2027 report delivers an in-depth understanding of market trends, market drivers, market barriers, and key tissue heart valves companies, including Abbott, Boston Scientific Corporation, CryoLife, Inc., Edward Lifesciences Corporation, Medtronic, among others.
Other Trending Reports
Treatment Resistant Depression Market | Advanced Renal Cell Carcinoma Market | Brain Cancer Market | Centronuclear Myopathy Market | Head And Neck Squamous Cell Carcinoma Market | Acquired Immunodeficiency Syndrome Market | Acute Intermittent Porphyria Market | Neurovascular Devices Market | Defibrillators Market | Ventricular Hypertrophy Market | Urolithiasis Market | Alopecia Areata Market | Autonomic Dysfunction Market | Acute Ischemic Stroke Ais Market | Adenoid Cystic Carcinoma Market | Aspergillosis Market | Biliary Atresia Market | Biliary Tumor Market | Chronic Inducible Urticaria Market | Chronic Insomnia Market | Critical Limb Ischemia Market | Endometriosis Pain Market | Generalized Anxiety Disorder Gad Market | Hallux Valgus Market | Hemophilia B Market | Immunologic Deficiency Syndrome Market | Neuroblastoma Market | Neuromodulation Devices Market | Neurovascular Thrombectomy Devices Market | Osteosarcoma Market | Pemphigus Vulgaris Market | Progressive Familial Intrahepatic Cholestasis Market | Pruritus Market | Radiation Toxicity Market | Pulmonary Hypertension Associated With Interstitial Lung Disease Market | Cluster Headaches Market | Foot And Ankle Devices Market | Intravenous Immunoglobulin Market | Bile Duct Neoplasm Market | Blood Glucose Monitoring Systems Market | Rett Syndrome Market | Tissue Heart Valves Market | Cardiac Biomarkers Testing Devices Market | Subscription Healthcare | Hepatorenal Syndrome Market | Central Venous Catheters Market | Continuous Renal Replacement Therapy Machines Market | Nonalcoholic Steatohepatitis Market | Necrotizing Enterocolitis Market | Cardiac Amyloidosis Market | Artificial Iris Market | Aortic Aneurysm Stent Grafts Market | Polypoidal Choroidal Vasculopathy Market | Adrenal Crisis Market | Hearing Implants Market | Image Guided Surgery Devices Market | Angioedema Market | Bladder Cancer Market
Related Healthcare Services
Healthcare Competitive Intelligence Services
Healthcare Asset Prioritization Services
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance.

Artificial Intelligence
Data Center Chip Market Size was Valued at USD 11.7 Billion in 2022 and is Expected to Reach USD 45.3 Billion by 2032 at a CAGR of 14.6% | Valuates Reports
BANGALORE, India, July 26, 2024 /PRNewswire/ — Data Center Chip Market By Chip Type (GPU, ASIC, FPGA, CPU, Others), By Data Center Size (Small and Medium Size, Large Size), By Industry Verticals (BFSI, Manufacturing, Government, IT and Telecom, Retail, Transportation, Energy and Utilities, Others): Global Opportunity Analysis and Industry Forecast, 2023-2032.
The Data Center Chip Market was valued at USD 11.7 Billion in 2022, and is estimated to reach USD 45.3 Billion by 2032, growing at a CAGR of 14.6% from 2023 to 2032.
Get Free Sample @ https://reports.valuates.com/request/sample/ALLI-Auto-2B326/Data_Center_Chip_Market
Major Factors Driving the Growth of Data Center Chip Market
Because of the growing need for data processing and storage solutions brought about by the quick development of cloud computing, artificial intelligence, and big data analytics, the data center chip market is expanding significantly. High-performance chips are necessary for data centers to process massive volumes of data quickly and efficiently. As a result, advances in chip technology, including CPUs, GPUs, and specialist AI processors, have been made. The need for more resilient and scalable data center infrastructure is fueled in part by the expansion of digital services and Internet of Things (IoT) devices. The market is expanding due to key areas including Asia-Pacific, with its investments in technology and fast digital transformation, and North America, with its top tech businesses and vast data center networks.
View Full Report @ https://reports.valuates.com/market-reports/ALLI-Auto-2B326/data-center-chip
TRENDS INFLUENCING THE GROWTH OF THE DATA CENTER CHIP MARKET:
In data centers, Graphics Processing Units (GPUs) are essential for speeding up computing operations and data processing. They are perfect for managing workloads related to artificial intelligence (AI), machine learning, and large-scale data analytics because of their parallel processing capabilities. The need for GPUs in data centers is growing as these technologies become increasingly essential to corporate operations. Businesses are purchasing GPUs in order to increase the effectiveness of their data processing, lower latency, and boost overall performance. The need for data center chips is being driven by the increasing reliance on GPUs for sophisticated computing activities, which is considerably contributing to the market’s rise. This need is further increased by the growing use of AI and machine learning in a variety of sectors, which puts GPUs at the forefront of the data center semiconductor industry.
Compared to general-purpose chips, Application Specific Integrated Circuits (ASICs) provide better performance and efficiency since they are designed specifically for a given application. ASICs are extensively utilized in data centers for specific tasks including networking, data compression, and encryption. ASICs are becoming more and more common as a result of the growth of cloud computing, big data analytics, and blockchain technology, which has increased demand for high-performance, energy-efficient processors. Their capacity to provide tailored performance for certain applications aids data centers in better workload management, power conservation, and operating expense reduction. The market is expanding as a result of the increased preference for ASICs in data centers, which is fueling the need for specialized data center chips.
Large data centers are important users of data center chips; they are run by well-known IT firms and cloud service providers. To manage enormous volumes of data and provide a wide range of services, these facilities need a great deal of processing power and sophisticated computing skills. High-performance data center chips are becoming more and more necessary as a result of the growth of massive data centers and the rising demand for online streaming, cloud services, and digital transactions. These chips are necessary to ensure effective data management, processing, and storage, which helps big data centers fulfill the increasing expectations of its clientele. Large data center proliferation is anticipated to considerably boost the data center chip industry as the digital economy continues to grow.
Data centers are becoming more and more important to the Banking, Financial Services, and Insurance (BFSI) industry as a means of safely and effectively managing high transaction volumes, consumer data, and financial records. The need for sophisticated data center processors is being driven by the sector’s requirement for real-time data processing, high-performance computing, and strong security measures. BFSI organizations may improve their operational efficiency, guarantee data integrity, and deliver superior client services by utilizing data centers fitted with robust chips. The BFSI sector’s need for data center chips is being driven by the increasing use of online banking, digital banking, and financial analytics tools, all of which increase the requirement for sophisticated data center infrastructure.
The market for data center chips is significantly influenced by the cloud computing industry’s explosive growth. There is a growing need for scalable, effective, and high-performance data center infrastructure as more companies move their operations to the cloud. In order to handle enormous volumes of data, facilitate virtualization, and guarantee flawless service delivery, cloud service providers need sophisticated data center chips. Sturdy data center chips are becoming more and more necessary as cloud-based solutions become more and more popular. Benefits like cost savings, flexibility, and scalability are driving this trend. In places like North America and Europe, where cloud adoption rates are high and data center chip demand is rising rapidly, this tendency is especially significant.
Buy Now @ https://reports.valuates.com/api/directpaytoken?rcode=ALLI-Auto-2B326&lic=single-user
DATA CENTER CHIP MARKET SHARE
In 2022, North America gained a sizable portion of the market.
In 2022, the GPU made up the largest portion of the market share.
Throughout the projection period, large data centers are expected to gain a significant portion.
The BFSI market is anticipated to be one of the most profitable markets.
Purchase Regional Report @ https://reports.valuates.com/request/regional/ALLI-Auto-2B326/Data_Center_Chip_Market
Key Companies:
Advanced Micro Devices IncTaiwan Semiconductor Manufacturing Company LimitedBroadcomHuawei Technologies Co LtdIntel CorporationNVidia CorporationSamsung Electronics Co LtdQualcomm Technologies IncGlobalFoundriesARM LIMITED (SOFTBANK GROUP CORP.)Purchase Chapters @ https://reports.valuates.com/request/chaptercost/ALLI-Auto-2B326/Data_Center_Chip_Market
SUBSCRIPTION
We have introduced a tailor-made subscription for our customers. Please leave a note in the Comment Section to know about our subscription plans.
DISCOVER MORE INSIGHTS: EXPLORE SIMILAR REPORTS!
– The global modular data center market size was valued at USD 14,952 Million in 2019 and is projected to reach USD 59,971 Million by 2027, registering a CAGR of 18.7% from 2020 to 2027.
– Data Centre Market was estimated to be worth USD 137500 Million in 2023 and is forecast to a readjusted size of USD 412740 Million by 2030 with a CAGR of 16.8% during the forecast period 2024-2030.
– Data Center PCIe Chip market was valued at USD 194.9 Million in 2023 and is anticipated to reach USD 377.4 Million by 2030, witnessing a CAGR of 10.2% during the forecast period 2024-2030.
– Data Center Accelerator Market
– Data Center Networking Market
– According to a new report published by , titled, “Big Data Analytics in Semiconductor & Electronics Market,” The big data analytics in semiconductor & electronics market was valued at D18.7 billion in 2021, and is estimated to reach D47.2 billion by 2031, growing at a CAGR of 9.9% from 2022 to 2031.
– IoT market was valued at USD 34250 Million in 2023 and is anticipated to reach USD 74630 Million by 2030, witnessing a CAGR of 11.6% during the forecast period 2024-2030.
– Data Center AI Accelerator Chip Market
– Electro-absorption Modulated Laser Chip Market
– According to a new report published by , titled, “Data Processing Unit Market”, the data processing unit market was valued at D553.96 Million in 2021, and is estimated to reach D5.5 billion by 2031, growing at a CAGR of 26.9% from 2022 to 2031.
– Optical Chip for Data Center Market
– EML Chip Market
– Optical Communication Chip Market revenue was USD 3102.7 Million in 2022 and is forecast to a readjusted size of USD 7251.5 Million by 2029 with a CAGR of 12.9% during the forecast period (2023-2029).
– SiC Power Chip Market
– Silicon Carbide Chip Market
– High Speed Optical Communication Chip Market
– 56G eml Optical Chip Market
– FPGA Master Control Chip Market
– Single-port Industrial Grade Ethernet PHY Chip Market
– AWG Chip Market
– Discrete Graphics Chip Market
– LNOI Optoelectronic Chip Market
– Smartphone Chip Market
– Smartphone TFT-LCD Display Driver Chip Market
– Smart Phone Baseband Chip Market
DISCOVER OUR VISION: VISIT ABOUT US!
Valuates offers in-depth market insights into various industries. Our extensive report repository is constantly updated to meet your changing industry analysis needs.
Our team of market analysts can help you select the best report covering your industry. We understand your niche region-specific requirements and that’s why we offer customization of reports. With our customization in place, you can request for any particular information from a report that meets your market analysis needs.
To achieve a consistent view of the market, data is gathered from various primary and secondary sources, at each step, data triangulation methodologies are applied to reduce deviance and find a consistent view of the market. Each sample we share contains a detailed research methodology employed to generate the report. Please also reach our sales team to get the complete list of our data sources.
YOUR FEEDBACK MATTERS: REACH OUT TO US!Valuates [email protected] U.S. Toll-Free Call 1-(315)-215-3225WhatsApp: +91-9945648335Website: https://reports.valuates.comBlog: https://valuatestrends.blogspot.com/ Pinterest: https://in.pinterest.com/valuatesreports/ Twitter: https://twitter.com/valuatesreports Facebook: https://www.facebook.com/valuatesreports/ YouTube: https://www.youtube.com/@valuatesreports6753 https://www.facebook.com/valuateskorean https://www.facebook.com/valuatesspanish https://www.facebook.com/valuatesjapanese https://valuatesreportspanish.blogspot.com/ https://valuateskorean.blogspot.com/ https://valuatesgerman.blogspot.com/ https://valuatesreportjapanese.blogspot.com/
Logo: https://mma.prnewswire.com/media/1082232/Valuates_Reports_Logo.jpg
View original content:https://www.prnewswire.co.uk/news-releases/data-center-chip-market-size-was-valued-at-usd-11-7-billion-in-2022-and-is-expected-to-reach-usd-45-3-billion-by-2032-at-a-cagr-of-14-6–valuates-reports-302207745.html
Artificial Intelligence
Industry 4.0 Market to Surpass USD 513.89 Billion by 2031 with Automation Surge | SkyQuest Technology
WESTFORD, Mass., July 26, 2024 /PRNewswire/ — According to SkyQuest, the global Industry 4.0 Market size was valued at USD 133.05 billion in 2022 and is poised to grow from USD 154.6 billion in 2023 to USD 513.89 billion by 2031, growing at a CAGR of 16.2% during the forecast period (2024-2031).
Industry 4.0 or the fourth industrial revolution emphasizes the use of automation and interconnectivity. Employment of advanced technologies such as artificial intelligence, machine learning, robotics, and connected devices to improve the productivity and efficiency of industries. Rapid digitization and advancements in technology are forecasted to bolster the Industry 4.0 market growth over the coming years. The global Industry 4.0 market is segmented into technology, industry vertical, and region.
Download a detailed overview:
https://www.skyquestt.com/sample-request/industry-4-0-market
Industry 4.0 Market Overview:
Report Coverage
Details
Market Revenue in 2023
$ 154.6 billion
Estimated Value by 2031
$ 513.89 billion
Growth Rate
Poised to grow at a CAGR of 16.2%
Forecast Period
2024–2031
Forecast Units
Value (USD Billion)
Report Coverage
Revenue Forecast, Competitive Landscape, Growth Factors, and Trends
Segments Covered
Technology, Industry and Region
Geographies Covered
North America, Europe, Asia Pacific, Latin America, and Middle East and Africa.
Report Highlights
Internet of Things (IoT) technology takes centerstage for Industry 4.0 adoption
Key Market Opportunities
Adoption of smart manufacturing and additive manufacturing practices
Key Market Drivers
Rising demand for automation across all industry verticals
Segments covered in Industry 4.0 Market are as follows:
TechnologyRobots (Traditional Industrial Robots {Articulated robots, Cartesian Robots, Selective Compliance Assembly Robot Arm (SCARA), Cylindrical Robots, Others}, Collaborative Robots), Blockchain in Manufacturing, Industrial Sensors (Level Sensors, Temperature Sensors, Flow Sensors, Position Sensors, Pressure Sensors, Force Sensors, Humidity & Moisture Sensors, Gas Sensors), Industrial 3D Printing, Machine Vision (Camera {Digital Camera, Smart Camera}, Frame Grabbers, Optics, and LED Lighting, Processor and Software), HMI (Offering {Hardware [Basic HMI, Advanced Panel-based HMI, Advanced PC-based HMI, Others], Software [On-premises HMI, Cloud-based HMI], Services}), Configuration ({Embedded HMI, Standalone HMI}, Technology {Motion HMI, Bionic HMI, Tactile HMI, Acoustic HMI}, End-user Industry {Process industries [Oil & Gas, Food & beverages, Pharmaceuticals, Chemicals, Energy & power, Metals & mining, Water & wastewater, Others], Discrete industry [Automotive, Aerospace & defense, Packaging, Medical devices, Semiconductor & electronics, Others]}), AI In Manufacturing (Offering {Hardware [Processor MPU, GPU, FPGA, ASIC, Memory, Network], Software [AI solutions- | On-premises, Cloud |, AI platform- | Machine learning framework, Application program interface |], Services [Deployment & integration, Support & maintenance]}, Technology {Machine learning [Deep learning, Supervised learning, Reinforcement learning, Reinforcement learning, Others], Natural language processing [Context-aware computing, Computer vision]}, Application {Predictive maintenance and machinery inspection, Material movement, Production planning, Field services, Quality control, Cybersecurity, Industrial robots, Reclamation}, Digital Twin {Technology [Internet of Things (IOT), Blockchain, Artificial intelligence & machine learning, Artificial intelligence & machine learning, Big data analytics, 5G], Usage Type [Product digital twin, Process digital twin, System digital twin], Application [Product design & development, Performance monitoring, Predictive maintenance, Inventory management, Business optimization, Others]}, Automated Guided Vehicles (AGV) {Type [Tow vehicles, Unit load carriers, Pallet trucks, Assembly line vehicles, Forklift trucks, Others], Navigation Technology [Laser guidance, Magnetic guidance, Inductive guidance, Optical tape guidance, Vision guidance, Others]}, Machine Condition Monitoring {Monitoring Technique [Vibration monitoring, Embedded systems, Vibration analyzers and meters, Thermography, Oil analysis, Corrosion monitoring, Ultrasound emission, Motor current analysis], Offering [Hardware – Vibration sensors, Accelerometers, Tachometers, Infrared sensors, Spectrometers, Ultrasound detectors, Spectrum analyzers, Corrosion probes], Software [Data integration, Diagnostic reporting, Order tracking analysis, Parameter calculation], Deployment Type [On-premises deployment, Cloud deployment], Monitoring Process [Online condition monitoring, Portable condition monitoring]})IndustryManufacturing, Automotive, Energy, Medical, Semiconductor & Electronics, Food & Beverage, Oil & Gas, Aerospace, Metals & Mining, Chemicals, and OthersRequest Free Customization of this report:
https://www.skyquestt.com/speak-with-analyst/industry-4-0-market
Internet of Things (IoT) Technology to Remain Indispensable for Industry 4.0
Internet of Things (IoT) remains the most crucial technology in global Industry 4.0 market growth owing to its role in interconnectivity and automation across different verticals. Advancements in connectivity technologies and rising use of automation in different industry verticals are also estimated to help this sub-segment gain an impressive market share. Surging demand for predictive maintenance will also boost the adoption of IoT technology in the long run.
Advanced robotic technologies are also slated to gain traction in the Industry 4.0 market. Growing acceptance of robots and high investments in advancements of robotic technologies are also slated to create new opportunities for providers of advanced robotics in the Industry 4.0 market. The low margin of error and the immense scope of automation are key benefits of robotics that help this sub-segment flourish.
Artificial intelligence (AI) will be another popular technology in the Industry 4.0 world going forward. Increasing demand for continuous monitoring, real-time analytics, and predictive maintenance are slated to help the demand for artificial intelligence in the future. The rising use of IoT devices will also boost the demand for cloud computing technology in the long run.
View report summary and Table of Contents (TOC):
https://www.skyquestt.com/report/industry-4-0-market
Manufacturing Vertical to Spearhead Industry 4.0 Market Development
The manufacturing vertical is estimated to be at the forefront when it comes to Industry 4.0 adoption. The surge in use of robotics, advanced technologies, and smart manufacturing practices sets the tone for Industry 4.0 in this industry vertical. High emphasis on improving manufacturing efficiency, reducing downtime, and maximizing profits are all contributing to the high market share of this sub-segment.
The automotive industry is another vertical where Industry 4.0 market players could invest to get good returns. The high adoption of advanced robotics and other smart manufacturing technologies to maximize production allows this sub-segment to become a crucial one for Industry 4.0 providers. The aerospace and defense industry vertical also shows a lot of promise for Industry 4.0 companies going forward. Growing demand for advanced manufacturing techniques and technologies to create complex aerospace components is helping Industry 4.0 market growth via this segment.
The oil & gas industry is also estimated to embrace Industry 4.0 trend with open hands as they try to improve their operations and promote better resource utilization. High demand for predictive maintenance to reduce downtime and the growing adoption of digital oilfield solutions are estimated to bolster Industry 4.0 market development in the long run.
To sum it up, the application scope for Industry 4.0 is endless as automation and digitization pick up pace around the world. High investments in development of IoT and AI technologies will create better opportunities for Industry 4.0 companies in the future. The manufacturing industry will remain the top revenue generating sub-segment and more opportunities for aerospace, automotive, and oil & gas verticals will be seen over the coming years.
Related Report:
Digital Twin Market
Cyber Security Market
Artificial Intelligence (AI) Market
Internet Of Things (IoT) Market
Machine Learning Market
About Us:
SkyQuest is an IP focused Research and Investment Bank and Accelerator of Technology and assets. We provide access to technologies, markets and finance across sectors viz. Life Sciences, CleanTech, AgriTech, NanoTech and Information & Communication Technology.
We work closely with innovators, inventors, innovation seekers, entrepreneurs, companies and investors alike in leveraging external sources of R&D. Moreover, we help them in optimizing the economic potential of their intellectual assets. Our experiences with innovation management and commercialization has expanded our reach across North America, Europe, ASEAN and Asia Pacific.
Contact: Mr. Jagraj SinghSkyQuest Technology1 Apache Way,Westford,Massachusetts 01886USA (+1) 351-333-4748Email: [email protected] Our Website: https://www.skyquestt.com/
Logo : https://mma.prnewswire.com/media/2446095/SkyQuest_Logo.jpg
View original content:https://www.prnewswire.co.uk/news-releases/industry-4-0-market-to-surpass-usd-513-89-billion-by-2031-with-automation-surge–skyquest-technology-302206499.html
Artificial Intelligence
Generative AI Cybersecurity Market worth $40.1 billion by 2030 – Exclusive Report by MarketsandMarkets™
CHICAGO, July 26, 2024 /PRNewswire/ — The Generative AI cybersecurity Market is anticipated to experience substantial expansion, ascending from a value of USD 7.1 billion in 2024 to a substantial worth of USD 40.1 billion by the year 2030, according to a new report by MarketsandMarkets™. This growth trajectory reflects a robust compound annual growth rate (CAGR) of 33.4% over the forecast period.
Browse in-depth TOC on “Generative AI cybersecurity Market”
350 – Tables 60 – Figures450 – Pages
Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=164202814
Scope of the Report
Report Metrics
Details
Market size available for years
2019–2030
Base year considered
2023
Forecast period
2024–2030
Forecast units
USD (Million)
Segments Covered
Offering, Generative AI-based Cybersecurity, Cybersecurity for Generative AI, Security Type, End-user, and Region
Geographies covered
North America, Europe, Asia Pacific, Middle East & Africa, and Latin America
Companies covered
Microsoft (US), IBM (US), Google (US), SentinelOne (US), AWS (US), NVIDIA (US), Cisco (US), CrowdStrike (US), Fortinet (US), Zscaler (US), Trend Micro (Japan), Palo Alto Networks (US), BlackBerry (Canada), Darktrace (UK), F5 (US), Okta (US), Sangfor (China), SecurityScorecard (US), Sophos (UK), Broadcom (US), Trellix (US), Veracode (US), LexisNexis (US), Abnormal Security (US), Adversa AI (Israel), Aquasec (US), BigID (US), Checkmarx (US), Cohesity (US), Credo AI (US), Cybereason (US), DeepKeep (Israel), Elastic NV (US), Flashpoint (US), Lakera (US), MOSTLY AI (Austria), Recorded Future (US), Secureframe (US), Skyflow (US), SlashNext (US), Snyk (US), Tenable (US), TrojAI (Canada), VirusTotal (Spain), XenonStack (UAE), and Zerofox (US).
This dramatic surge is being fueled by a number of causes. The primary growth driver is the enhancement of existing cybersecurity tools through generative AI algorithms by improving anomaly detection, automating threat hunting and penetration testing, and providing complex simulations for security testing purposes. These techniques enable various cyber-attack scenarios that can be simulated using the Generative Adversarial Networks (GANs), thus enabling the development of better preparedness and response strategies. On the other hand, it requires special cyber security tools to protect generative AI workloads against unique vulnerabilities such as adversarial attacks, model inversions and LLM poisoning. These tools include differential privacy and secure multi-party computation that are integrated into AI systems for training and deployment data protection purposes.
Request Sample Pages@ https://www.marketsandmarkets.com/requestsampleNew.asp?id=164202814
Generative AI apps security segment will account for largest market share during the forecast period.
The cybersecurity landscape is rapidly changing for generative AI apps, which are already making their way into chatbots, content creation tools like word processors, and personalized recommendation systems. According to McAfee, 55% of these programs have had security breaches. This highlights the dire need for stronger protective measures from unauthorized access. Several generative AI applications that use adversarial techniques to force the desired reaction out of intelligent machines.
Therefore, there is a pressing demand in the number of developers who ensure that such machines are made more robust through techniques like adversarially trained models and resistant architectures. Finally, the usage of secure enclaves plus hardware-based security measures is growing off late, mainly aimed at safeguarding vulnerable AI computations from being tampered with. For instance, OpenAI has very strict security rules meant to protect GPT models thereby ensuring data integrity and user privacy.
By end-user, government & defense sector is poised to account for larger market share in 2024.
Government as well as defense industries are increasingly resorting to generative AI for cyber security purposes due to the urgency of protecting sensitive information and national security. According to a recent CSIS report, AI is being integrated into the cybersecurity framework of 43% of government agencies which resultantly improves their ability to identify and counter threats. As an example, the United States Department of Defense has started using artificial intelligence (AI) based security solutions backed by generative AI that can create fictitious cyber-attacks, thereby providing them with enhanced preparedness against advanced types of threats.
This technology also helps these sectors handle and analyze large volumes of data more effectively, giving valuable insights that will enable them prevent or mitigate cyber threats. This trend demonstrates an increasing reliance on generative AI in fortifying cyber security measures so as to ensure that critical infrastructure and sensitive data remain secure in today’s intricate digital landscape.
By region, North America to hold the largest share by market value in 2024.
In 2024, North America will be the leading region based on market share due to its excellent technology infrastructure, substantial investments in AI-enabled cybersecurity and the presence of key players. Major cyber security research universities and tech companies such as Google, AWS, CrowdStrike, SentinelOne and IBM are present in this area, pushing them on the forefront of potent risk management technologies and generative AI tools for threat detection. For example, IBM’s security platform powered by AI has improved detection rates for threats up by 40%, thus proving the relevance of AI technology to enhancing cybersecurity.
Moreover, legislative instruments such as Cybersecurity Information Sharing Act (CISA) are being put in place to promote advanced cybersecurity technologies. As internet attacks continue getting more complicated, North American enterprises prefer generative artificial intelligence (AI), so as to enhance their safety measures pertaining to personal data and digital infrastructure.
Inquire Before Buying@ https://www.marketsandmarkets.com/Enquiry_Before_BuyingNew.asp?id=164202814
Top Key Companies in Generative AI cybersecurity Market:
The major players in the generative AI cybersecurity market include Palo Alto Networks (US), AWS (US), CrowdStrike (US), SentinelOne (US), and Google (US), along with SMEs and startups such as MOSTLY AI (Austria), XenonStack (UAE), BigID (US), Abnormal Security (US), and Adversa AI (Israel).
Browse Adjacent Market: Artificial Intelligence (AI) Market Research Reports & Consulting
Browse Other Reports:
AI Model Risk Management Market – Global Forecast to 2029
AI in Chemicals Market – Global Forecast to 2029
Artificial Intelligence in Cybersecurity Market – Global Forecast to 2028
Explainable AI Market – Global Forecast to 2028
Artificial Intelligence (AI) Toolkit Market – Global Forecast to 2028
Get access to the latest updates on Generative AI cybersecurity Companies and Generative AI cybersecurity Industry
About MarketsandMarkets™
MarketsandMarkets™ has been recognized as one of America’s best management consulting firms by Forbes, as per their recent report.
MarketsandMarkets™ is a blue ocean alternative in growth consulting and program management, leveraging a man-machine offering to drive supernormal growth for progressive organizations in the B2B space. We have the widest lens on emerging technologies, making us proficient in co-creating supernormal growth for clients.
Earlier this year, we made a formal transformation into one of America’s best management consulting firms as per a survey conducted by Forbes.
The B2B economy is witnessing the emergence of $25 trillion of new revenue streams that are substituting existing revenue streams in this decade alone. We work with clients on growth programs, helping them monetize this $25 trillion opportunity through our service lines – TAM Expansion, Go-to-Market (GTM) Strategy to Execution, Market Share Gain, Account Enablement, and Thought Leadership Marketing.
Built on the ‘GIVE Growth’ principle, we work with several Forbes Global 2000 B2B companies – helping them stay relevant in a disruptive ecosystem. Our insights and strategies are molded by our industry experts, cutting-edge AI-powered Market Intelligence Cloud, and years of research. The KnowledgeStore™ (our Market Intelligence Cloud) integrates our research, facilitates an analysis of interconnections through a set of applications, helping clients look at the entire ecosystem and understand the revenue shifts happening in their industry.
To find out more, visit www.MarketsandMarkets™.com or follow us on Twitter, LinkedIn and Facebook.
Contact:Mr. Rohan SalgarkarMarketsandMarkets™ INC.630 Dundee RoadSuite 430Northbrook, IL 60062USA: +1-888-600-6441Email: [email protected] Our Website: https://www.marketsandmarkets.com/
Logo: https://mma.prnewswire.com/media/1951202/4609423/MarketsandMarkets.jpg
View original content:https://www.prnewswire.co.uk/news-releases/generative-ai-cybersecurity-market-worth-40-1-billion-by-2030—exclusive-report-by-marketsandmarkets-302207361.html
-

 Artificial Intelligence3 days ago
Artificial Intelligence3 days agoaelf Welcomes First COO to Foster AI and Blockchain Synergies
-

 Artificial Intelligence3 days ago
Artificial Intelligence3 days agoSINGAPORE INKS MOU WITH QUANTINUUM, ENABLING ACCESS TO THEIR ADVANCED QUANTUM COMPUTER
-

 Artificial Intelligence3 days ago
Artificial Intelligence3 days agoDeep North Rebrands as StrataVision
-

 Artificial Intelligence3 days ago
Artificial Intelligence3 days agoCandyspace strengthens ecommerce capabilities with the acquisition of Profound
-

 Artificial Intelligence3 days ago
Artificial Intelligence3 days agoEmmes Group partners with Miimansa AI to accelerate adoption of Generative AI in clinical research
-

 Artificial Intelligence5 days ago
Artificial Intelligence5 days agoWego Announces Partnership with Visit Valencia to Promote the Spanish City as a Premier Travel Destination
-

 Artificial Intelligence3 days ago
Artificial Intelligence3 days agoAppdome Tackles Geo-Fraud with More Defenses for Mobile Apps
-

 Artificial Intelligence4 days ago
Artificial Intelligence4 days agoMedical & Commercial International (MCI) to Utilize GATC Health’s AI Platform to Launch World’s First Insurance-Backed Program to Finance Clinical Trials





















