Artificial Intelligence
Propulsion of Neuroendocrine Tumors Pipeline as Novel and Extensive 60+ Therapies Likely to Enter in the Treatment Domain | DelveInsight
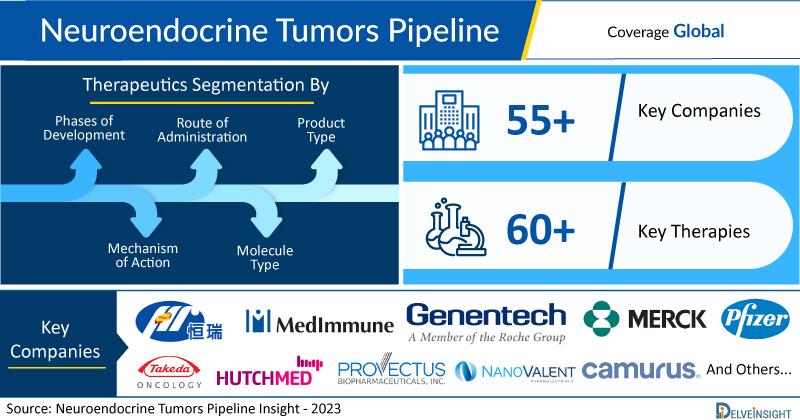
New York, USA, May 29, 2023 (GLOBE NEWSWIRE) — Propulsion of Neuroendocrine Tumors Pipeline as Novel and Extensive 60+ Therapies Likely to Enter in the Treatment Domain | DelveInsight
The prevalence of Neuroendocrine Tumors has been rising over the past few years, which prompts the growing demand for treatment options. The increasing prevalence of Neuroendocrine tumors and the rising awareness regarding the disease and its advancement in treatment among the population is helping to drive the market. The companies developing the potential therapies in the last stage of development include ITM Solucin, Pfizer, Boehringer Ingelheim, and several others.
DelveInsight’s ‘Neuroendocrine Tumors Pipeline Insight 2023‘ report provides comprehensive global coverage of pipeline neuroendocrine tumors therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the neuroendocrine tumors pipeline domain.
Key Takeaways from the Neuroendocrine Tumors Pipeline Report
- DelveInsight’s neuroendocrine tumors pipeline report depicts a robust space with 55+ active players working to develop 60+ pipeline therapies for neuroendocrine tumors treatment.
- Key neuroendocrine tumors companies such as Jiangsu Hengrui Medicine, MedImmune, Genentech, Merck & Co, Pfizer, Takeda Oncology, Hutchison MediPharma, Provectus Biopharmaceuticals, NanoValent Pharmaceuticals, Camurus, ITM Solucin, Pfizer, Radiomedix, Inc., Orano Med LLC, Clarity Pharmaceuticals, TaiRx, Peloton Therapeutics, RayzeBio, Ascentage Pharma, Debiopharm, Boehringer Ingelheim, Roche, Ipsen, Medelis Inc., Exelixis, EpicentRx, Inc., Tarveda Therapeutics, Teclison Ltd., Novatek Pharmaceuticals, Phanes Therapeutics, Fujifilm Pharmaceuticals, Jazz Pharmaceuticals, 23andMe, Inc., Celgene, Crinetics Pharmaceuticals Inc., POINT Biopharma, and others are evaluating new neuroendocrine tumors drugs to improve the treatment landscape.
- Promising neuroendocrine tumors pipeline therapies such as 177Lu-edotreotide PRRT, Axitinib, AlphaMedix, 64Cu MeCOSar Octreotate, Foslinanib, Belzutifan, Actinium-225 Dotate, Pelcitoclax, Debio 4126 controlled release, BI 764532, RO7616789, Satoreotide trizoxetan, rSIFN-co, CAM-2029, RRx-001, Lenvatinib, PEN-221, Belzutifan, Tirapazamine, Sunitinib, NP-101, PT217, FF-10850, Lurbinectedin, 23ME-00610, BLU-667, CC-223, Paltusotine, TAK 580, PV-10, NV 103, and others are under different phases of neuroendocrine tumors clinical trials.
- In March 2023, RayzeBio, Inc., a targeted radiopharmaceutical company developing an innovative pipeline against validated solid tumor targets, announced the completion of enrollment in the Phase 1b portion of the ACTION-1 Phase 1b/3 trial of RYZ101 in patients with SSTR+ gastroenteropancreatic neuroendocrine tumors (GEP-NETs) who have progressed on Lutetium-177 labeled somatostatin analog therapy.
- In March 2023, NUCLIDIUM announced that the Neuroendocrine Tumors Research Foundation (“NETRF”) has selected the company and its collaboration partner the University Hospital Basel (Universitätsspital Basel, “USB”) as recipients of its Investigator Award. The grant will support the initiation and execution of Phase 1 clinical trial with TraceNETTM, a novel copper-based radio diagnostic for detecting neuroendocrine tumors (NET).
- In March 2023, PharmaLogic Holdings Corp. announced the signature of a Master Services Agreement with Viewpoint Molecular Targeting, Inc., a wholly-owned subsidiary of Perspective Therapeutics, Inc., for the development and production of theranostic candidates VMT-01 and VMT-α-NET. Radiopharmaceuticals are currently in development for the diagnosis and treatment of metastatic melanoma and neuroendocrine tumors (NETs), respectively.
- In January 2023, Viewpoint Molecular Targeting®, Inc., announced the first dosing of two neuroendocrine tumor patients with therapeutic intent. VMT-α-NET, which is being developed for the treatment and diagnosis of somatostatin receptor subtype 2 (SSTR2) expressing neuroendocrine tumors, was administered to the patients in early December. The dosed patients were diagnosed with confirmed-advanced somatostatin-expressing neuroendocrine tumors (NETs).
- In October 2022, the FDA granted a fast-track designation to 177Lu-edotreotide (ITM-11) for use as a potential therapeutic option in patients with gastroenteropancreatic neuroendocrine tumors (GEP-NETs), according to an announcement from ITM Isotype Technologies Munich SE. ITM-11 is comprised of the octreotide-derived somatostatin analog edreotide (Dotatoc) and no-carrier lutetium-177 chloride, which is a beta-emitting therapeutic radioisotope.
- In November 2022, Lantheus Holdings, Inc. and POINT Biopharma Global Inc. (“POINT”) (NASDAQ: PNT), a company accelerating the discovery, development, and global access to life-changing radiopharmaceuticals, announced a set of strategic collaboration agreements in which Lantheus will license exclusive worldwide rights(1) to POINT’s PNT2002 and PNT2003 product candidates.
Request a sample and discover the recent advances in neuroendocrine tumors treatment drugs @ Neuroendocrine Tumors Pipeline Report
The neuroendocrine tumors pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage neuroendocrine tumors drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the neuroendocrine tumors clinical trial landscape.
Neuroendocrine Tumors Overview
Neuroendocrine tumors are rare types that develop in specialized cells known as neuroendocrine cells. Neuroendocrine cells contain characteristics that are similar to nerve cells and hormone-producing cells. Neuroendocrine tumors can arise everywhere in the body, although most occur in the lungs, appendix, small intestine, rectum, and pancreas. Neuroendocrine tumors form when neuroendocrine cells’ DNA changes. The neuroendocrine tumors symptoms include pain from a growing tumor, a growing lump you can feel under the skin, loss of appetite/weight loss, losing weight without trying, skin flushing, diarrhea, and others.
A thorough physical examination and a battery of specialized tests are used to make neuroendocrine tumors diagnosis. Ultrasound, CT, and MRI imaging examinations aid in the diagnosis of tumor images. Images for neuroendocrine tumors are sometimes obtained utilizing positron emission tomography (PET) using a radioactive tracer injected into a vein. A biopsy can also be performed. Neuroendocrine tumor treatment is determined by the type of tumor, its location, and its severity. Chemotherapy, targeted medication therapy, and hormone therapy are all neuroendocrine tumors treatment possibilities. The tumor can be removed surgically.

Find out more about neuroendocrine tumors treatment drugs @ Drugs for Neuroendocrine Tumors Treatment
A snapshot of the Neuroendocrine Tumors Pipeline Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| 177Lu-edotreotide PRRT | ITM Solucin | Phase III | Ionizing radiation emitters | Intravenous |
| Axitinib | Pfizer | Phase II/III | Angiogenesis inhibitors; Platelet-derived growth factor beta receptor antagonists; Proto oncogene protein c-kit inhibitors; Vascular endothelial growth factor receptor 3 antagonists; Vascular endothelial growth factor receptor-1 antagonists; Vascular endothelial growth factor receptor-2 antagonists | Oral |
| AlphaMedix | Radiomedix, Inc./Orano Med LLC | Phase II | Ionising radiation emitters | Intravenous |
| 64Cu MeCOSar Octreotate | Clarity Pharmaceuticals | Phase II | Positron-emission tomography enhancers | Intravenous |
| Foslinanib | TaiRx | Phase II | Angiogenesis inhibitors; Apoptosis stimulants; Growth inhibitors | Oral |
| Belzutifan | Merck Sharp & Dohme LLC | Phase II | Endothelial PAS domain-containing protein 1 inhibitors | Oral |
| Actinium-225 Dotate | RayzeBio | Phase I/II | Ionising radiation emitters | NA |
| Pelcitoclax | Ascentage Pharma | Phase I | Bcl-X protein inhibitors; Proto-oncogene protein c-bcl-2 inhibitors | Intravenous |
| Debio 4126 controlled release | Debiopharm | Phase I | Growth hormone-releasing hormone inhibitors | Intramuscular |
| BI 764532 | Boehringer Ingelheim | Phase I | Antibody-dependent cell cytotoxicity; T lymphocyte stimulants | Intravenous |
| RO7616789 | Roche | Phase I | Undefined mechanism | Intravenous |
Learn more about the emerging neuroendocrine tumors pipeline therapies @ Neuroendocrine Tumors Clinical Trials
Neuroendocrine Tumors Therapeutics Assessment
The neuroendocrine tumors pipeline report proffers an integral view of the neuroendocrine tumors emerging novel therapies segmented by stage, product type, molecule type, mechanism of action, and route of administration.
Scope of the Neuroendocrine Tumors Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Intramuscular, Topical
- Therapeutics Assessment By Molecule Type: Monoclonal antibody, Peptides, Polymer, Small molecule, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Angiogenesis inhibitors, Platelet-derived growth factor beta receptor antagonists, Proto oncogene protein c-kit inhibitors, Vascular endothelial growth factor receptor 3 antagonists, Vascular endothelial growth factor receptor-1 antagonists, Vascular endothelial growth factor receptor-2 antagonists, Ionising radiation emitters, Bcl-X protein inhibitors, Proto-oncogene protein c-bcl-2 inhibitors, Positron-emission tomography enhancers, Apoptosis stimulants, Growth inhibitors, Growth hormone-releasing hormone inhibitors, Somatostatin receptor agonists, Endothelial PAS domain-containing protein 1 inhibitors, Antibody-dependent cell cytotoxicity, T lymphocyte stimulants
- Key Neuroendocrine Tumors Companies: Jiangsu Hengrui Medicine, MedImmune, Genentech, Merck & Co, Pfizer, Takeda Oncology, Hutchison MediPharma, Provectus Biopharmaceuticals, NanoValent Pharmaceuticals, Camurus, ITM Solucin, Pfizer, Radiomedix, Inc., Orano Med LLC, Clarity Pharmaceuticals, TaiRx, Peloton Therapeutics, RayzeBio, Ascentage Pharma, Debiopharm, Boehringer Ingelheim, Roche, Ipsen, Medelis Inc., Exelixis, EpicentRx, Inc., Tarveda Therapeutics, Teclison Ltd., Novatek Pharmaceuticals, Phanes Therapeutics, Fujifilm Pharmaceuticals, Jazz Pharmaceuticals, 23andMe, Inc., Celgene, Crinetics Pharmaceuticals Inc., POINT Biopharma, and others.
- Key Neuroendocrine Tumors Pipeline Therapies: 177Lu-edotreotide PRRT, Axitinib, AlphaMedix, 64Cu MeCOSar Octreotate, Foslinanib, Belzutifan, Actinium-225 Dotate, Pelcitoclax, Debio 4126 controlled release, BI 764532, RO7616789, Satoreotide trizoxetan, rSIFN-co, CAM-2029, RRx-001, Lenvatinib, PEN-221, Belzutifan, Tirapazamine, Sunitinib, NP-101, PT217, FF-10850, Lurbinectedin, 23ME-00610, BLU-667, CC-223, Paltusotine, TAK 580, PV-10, NV 103, and others.
Dive deep into rich insights for new drugs for neuroendocrine tumors treatment, visit @ Neuroendocrine Tumors Drugs
Table of Contents
| 1. | Neuroendocrine Tumors Pipeline Report Introduction |
| 2. | Neuroendocrine Tumors Pipeline Report Executive Summary |
| 3. | Neuroendocrine Tumors Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Neuroendocrine Tumors Clinical Trial Therapeutics |
| 6. | Neuroendocrine Tumors Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Neuroendocrine Tumors Pipeline: Late-Stage Products (Phase III) |
| 7.1. | 177Lu-edotreotide PRRT: ITM Solucin |
| 8. | Neuroendocrine Tumors Pipeline: Mid-Stage Products (Phase II) |
| 8.1. | Belzutifan: Merck Sharp & Dohme LLC |
| 9. | Neuroendocrine Tumors Pipeline: Early-Stage Products (Phase I) |
| 9.1. | BI 764532: Boehringer Ingelheim |
| 10. | Neuroendocrine Tumors Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Neuroendocrine Tumors Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Neuroendocrine Tumors Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the neuroendocrine tumors pipeline therapeutics, reach out @ Neuroendocrine Tumors Treatment Drugs
Related Reports
Neuroendocrine Tumors Epidemiology
Neuroendocrine Tumors Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology as well as the neuroendocrine tumors epidemiology trends.
Neuroendocrine Tumors Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key neuroendocrine tumors companies, including Provectus Biopharmaceuticals, NanoValent Pharmaceuticals, Provectus Biopharmaceuticals, Vivace Therapeutics, among others.
Gastroenteropancreatic Neuroendocrine Tumors Market
Gastroenteropancreatic Neuroendocrine Tumors Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key gastroenteropancreatic neuroendocrine tumors companies, including Novartis, Ipsen Biopharmaceuticals, Pfizer, ITM Isotopen Technologien Muenchen, Camurus AB, Hutchison Medipharma Limited, among others.
Gastroenteropancreatic Neuroendocrine Tumors Pipeline
Gastroenteropancreatic Neuroendocrine Tumors Pipeline Insight – 2023 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key gastroenteropancreatic neuroendocrine tumors companies, including Novartis, Ipsen Biopharmaceuticals, Pfizer, ITM Isotopen Technologien Muenchen, Camurus AB, Hutchison Medipharma Limited, among others.
Gastroenteropancreatic Neuroendocrine Tumors Epidemiology
Gastroenteropancreatic Neuroendocrine Tumors Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology as well as the gastroenteropancreatic neuroendocrine tumors epidemiology trends.
Other Trending Reports
Treatment Resistant Depression Market | Advanced Renal Cell Carcinoma Market | Brain Cancer Market | Centronuclear Myopathy Market | Head And Neck Squamous Cell Carcinoma Market | Acquired Immunodeficiency Syndrome Market | Acute Intermittent Porphyria Market | Neurovascular Devices Market | Defibrillators Market | Ventricular Hypertrophy Market | Urolithiasis Market | Alopecia Areata Market | Autonomic Dysfunction Market | Acute Ischemic Stroke Ais Market | Adenoid Cystic Carcinoma Market | Aspergillosis Market | Biliary Atresia Market | Biliary Tumor Market | Chronic Inducible Urticaria Market | Chronic Insomnia Market | Critical Limb Ischemia Market | Endometriosis Pain Market | Generalized Anxiety Disorder Gad Market | Hallux Valgus Market | Hemophilia B Market | Immunologic Deficiency Syndrome Market | Neuroblastoma Market | Neuromodulation Devices Market | Neurovascular Thrombectomy Devices Market | Osteosarcoma Market | Pemphigus Vulgaris Market | Progressive Familial Intrahepatic Cholestasis Market | Pruritus Market | Radiation Toxicity Market | Pulmonary Hypertension Associated With Interstitial Lung Disease Market | Cluster Headaches Market | Foot And Ankle Devices Market | Intravenous Immunoglobulin Market | Bile Duct Neoplasm Market | Blood Glucose Monitoring Systems Market | Rett Syndrome Market | Tissue Heart Valves Market | Cardiac Biomarkers Testing Devices Market | Subscription Healthcare | Hepatorenal Syndrome Market | Central Venous Catheters Market | Continuous Renal Replacement Therapy Machines Market | Nonalcoholic Steatohepatitis Market | Necrotizing Enterocolitis Market | Cardiac Amyloidosis Market | Artificial Iris Market | Aortic Aneurysm Stent Grafts Market | Polypoidal Choroidal Vasculopathy Market | Adrenal Crisis Market | Hearing Implants Market | Image Guided Surgery Devices Market | Angioedema Market | Bladder Cancer Market
Related Healthcare Services
Healthcare Competitive Intelligence Services
Healthcare Asset Prioritization Services
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Artificial Intelligence
BeeHero Reveals Groundbreaking Data-Backed Insights from the Latest Almond Pollination Seasons
On World Bee Day, BeeHero shares findings from the 2022-2024 almond pollination seasons that showcase its ability to optimize and boost pollination efficacy while decreasing operational costs, by empowering both beekeepers and growers with innovative, data-driven solutions
FRESNO, Calif., May 20, 2024 /PRNewswire/ — BeeHero, the pioneer of precision pollination, today unveiled groundbreaking data-driven bee activity insights from the 2022-2024 almond pollination seasons. The company has revealed bee flight hour and bee frame insights via its cutting-edge sensor technology and AI-powered analysis that contribute to improved pollination practices. In recent months, BeeHero cemented its position as the world’s leading provider of precision pollination as it surpassed the threshold of 300K hives under management, from which the company records more than 25 million hive samples daily. The new data is being released on World Bee Day, the date designated by the UN to raise awareness of the importance of these vital pollinators, the numerous threats they face, and how they contribute to sustainable agriculture and development.
Analysis of the previous almond seasons revealed that BeeHero-managed hives demonstrated a higher degree of effectiveness, with an average of nearly 50% more bee-frames per hive compared to the industry standard. This superior performance translates into stronger colonies and enables BeeHero to optimize hive placement and reduce the number of hives required per acre in almond orchards, resulting in enhanced pollination at a lower input-to-output ratio for growers.
BeeHero also discovered a significant difference in the average daily bee flight hours (BFH) measured by its sensors as compared to the traditional bee flight hour calculations. While conventional bee flight hour methods (based on industry standard hives) recorded a total of 2.7 daily BFH over the 2022 and 2023 almond pollination seasons, BeeHero was able to more accurately measure almost double this amount, at 5.9 daily BFH, demonstrating that bees will indeed fly in suboptimal conditions. This revelation underscores the higher accuracy of BeeHero’s methodology over traditional calculations, which underestimate the actual flying time of bees due to a reliance on and proximity to local national weather stations, affecting industry crop yield predictions. Both this finding and BeeHero’s ability to provide stronger hives have widespread implications for not only almonds, but other seed, row, and specialty crops as well.
“We are excited to be consistently achieving new, pivotal milestones on our mission to transform pollination efficiency through transparency and data-driven precision,” explained Omer Davidi, CEO and Co-Founder of BeeHero. “Our findings showcase the critical nature of robust data in optimizing pollination activities, and our unique ability to provide previously unknowable insights – and as a result, stronger hives and more accurate yield predictions – to industry stakeholders. We look forward to continuing to reshape industry paradigms, empowering growers and beekeepers to better foster bee welfare and bolstering productivity for greater profitability.”
During the 2024 almond pollination season, BeeHero utilized various proprietary tools to extract its unparalleled dataset on bee behavior and pollination efficacy. The company introduced a Deployment Planning Tool, enabling beekeepers to visualize their almond orchards and strategically plan daily tasks for maximum efficiency. Additionally, its Hive Tracker offered growers real-time insights into hive shipment and placement, while BeeHero’s new mobile growers platform provided growers with seamless access to hives’ frame counts and other critical information and updates.
Following the culmination of the pollination season, BeeHero is providing personalized precision pollination reports for growers with insights into bee flight hours, bee frames, and how BeeHero’s data and technology have directly impacted their season. In alignment with this mission, a recent study conducted by BeeHero and the USDA explored how bee colony strength and hive entrance orientation affected honey bee foraging behavior, offering actionable insights to improve pollination efficacy. The research underscores the pivotal role of BeeHero’s proprietary technology in gathering data to drive operational efficiency, reduce costs, increase yields, enhance bee welfare, and promote sustainable agriculture.
“The findings from these past pollination seasons – both in our research and in the field – highlight the profound potential of our innovative technology to revolutionize pollination practices, fostering a sustainable ecosystem that benefits both beekeepers and growers,” said Yuval Regev, CTO and Co-Founder of BeeHero. “By illuminating intricate bee behavior patterns and ecosystem dynamics, we are pioneering a new frontier in pollination science and technology.”
Earlier this month, BeeHero was recognized as an Honorable Mention by Fast Company’s 2024 World Changing Ideas Awards in the agriculture category. The award honors products and companies designed to make the world safer, cleaner, more sustainable, and more equitable. This is the latest in a slew of prestigious awards and recognitions for BeeHero, which include Cleantech Group’s 2024 Global Cleantech 100 list, the 2024 Founder’s Games for social impact, CNBC’s 2023 Disruptor 50 list, and The New York Times’ 2022 Good Tech Awards.
About BeeHero
BeeHero is a data-driven technology company redefining pollination in commercial agriculture. Using advanced data analytics, artificial intelligence, and low-cost IoT sensors, BeeHero brings transparency and efficiency to the complex logistics of commercial crop pollination. Its Precision Pollination as a Service (PPaaS) results in better crop yields and increased profits for commercial crop growers and agribusiness stakeholders. Its precision pollination solution is rapidly evolving into the backbone of the data-driven approach needed to build a resilient and future-proof sustainable agriculture ecosystem. The company is headquartered in Fresno, California, with offices in Palo Alto, California and R&D in Tel Aviv, Israel.
Media Contact
Allison GreyHeadline [email protected] US: +1 323 283 8176UK: +44 203 807 4482IL: +972 53 820 2606
View original content:https://www.prnewswire.co.uk/news-releases/beehero-reveals-groundbreaking-data-backed-insights-from-the-latest-almond-pollination-seasons-302150117.html
Artificial Intelligence
Dubai World Trade Centre Drives Impact as Economic Output Surges to US$4.98 Billion in 2023, up 40% YoY

DUBAI, UAE, May 20, 2024 /PRNewswire/ — Dubai World Trade Centre (DWTC), a global leader in the events and exhibitions industry, has once again demonstrated its significant impact on Dubai’s economy in 2023, welcoming 2.47 million participants and hosting 301 events, 76 of which, were large-scale events that attracted 1.54 million attendees, with 46% from overseas.
DWTC’s 2023 Economic Impact Assessment (EIA) Report, based on its 76 large-scale events (2000 or more attendees) revealed an impressive surge in the total economic output, reaching US$4.98 billion, marking an incredible 40% YoY increase, with high returns for adjacent industries such as Travel, Accommodation and Retail, connected to the Meetings Incentives Conferences and Exhibitions (MICE) ecosystem.
DWTC’s large-scale events generated a substantial US$2.87 billion Gross Value Added (GVA) to Dubai’s GDP, retaining an impressive 58% of the total economic output locally. International participation soared by 53%, with overseas visitors driving 6.2 times more contribution than domestic counterparts.
Events hosted at DWTC supported 69,281 jobs, generating US$915 million in disposable household income for the city’s residents. The substantial economic impact of these events extends beyond direct revenue generation, fostering socio-economic development and contributing to Dubai’s status as a leading global business hub.
His Excellency Helal Saeed Almarri, Director General of DWTC Authority, said: “Aligned with Dubai’s Economic Agenda D33, we continue to spearhead efforts in sector diversification, reinforcing the city’s stature as a leading global business hub. The remarkable accomplishments of 2023, presented in the ‘DWTC Economic Impact Assessment Report’ demonstrate that Dubai’s MICE sector, driven by DWTC, remains a vital pillar of financial resilience and growth underscoring our accelerated strides towards sustainable socio-economic development. The increase in international participation, along with the significant economic impact generated across diverse sectors such as travel, accommodation and retail, highlights the city’s steadfast commitment to propelling business tourism.”
The venue’s formidable events portfolio strategically aligned with Dubai’s economic priorities, showcasing Healthcare, Medical, and Scientific; Information Technology (IT); and Food, Hotel, and Catering as the top contributors. These leading sectors collectively accounted for 59% (US$1.71 billion) of the GVA to Dubai’s economy, and 49% (747,468) of the total large-scale event visitation.
Adjacent sectors, including hotels, air travel, and local transportation experienced a significant boost in economic activity. The direct revenue generated through expenditure was nearly US$2.94 billion.
Photo – https://mma.prnewswire.com/media/2416961/Dubai_World_Trade_Centre_2023_Infographic.jpg
View original content:https://www.prnewswire.co.uk/news-releases/dubai-world-trade-centre-drives-impact-as-economic-output-surges-to-us4-98-billion-in-2023–up-40-yoy-302150038.html
Artificial Intelligence
Aramco signs agreement with Pasqal to deploy first quantum computer in the Kingdom of Saudi Arabia
DHAHRAN, Saudi Arabia, May 20, 2024 /PRNewswire/ — Aramco, one of the world’s leading integrated energy and chemicals companies, has signed an agreement with Pasqal, a global leader in neutral atom quantum computing, to install the first quantum computer in the Kingdom of Saudi Arabia.
The agreement will see Pasqal install, maintain, and operate a 200-qubit quantum computer, which is scheduled for deployment in the second half of 2025.
Ahmad Al-Khowaiter, Aramco EVP of Technology & Innovation, said: “Aramco is delighted to partner with Pasqal to bring cutting-edge, high-performance quantum computing capabilities to the Kingdom. In a rapidly evolving digital landscape, we believe it is crucial to seize opportunities presented by new, impactful technologies and we aim to pioneer the use of quantum computing in the energy sector. Our agreement with Pasqal allows us to harness the expertise of a leading player in this field, as we continue to build state-of-the-art solutions into our business. It is also further evidence of our contribution to the growth of the digital economy in Saudi Arabia.”
Georges-Olivier Reymond, Pasqal CEO & Co-founder, said: “The era of quantum computing is here. No longer confined to theory, it’s transitioning to real-world applications, empowering organisations to solve previously intractable problems at scale. Since launching Pasqal in 2019, we have directed our efforts towards concrete quantum computing algorithms immediately applicable to customer use cases. Through this agreement, we’ll be at the forefront of accelerating commercial adoption of this transformative technology in Saudi Arabia. This isn’t just any quantum computer; it will be the most powerful tool deployed for industrial usages, unlocking a new era of innovation for businesses and society.”
The quantum computer will initially use an approach called “analog mode.” Within the following year, the system will be upgraded to a more advanced hybrid “analog-digital mode,” which is more powerful and able to solve even more complex problems.
Pasqal and Aramco intend to leverage the quantum computer to identify new use cases, and have an ambitious vision to establish a powerhouse for quantum research within Saudi Arabia. This would involve leading academic institutions with the aim of fostering breakthroughs in quantum algorithm development — a crucial step for unlocking the true potential of quantum computing.
The agreement also accelerates Pasqal’s activity in Saudi Arabia, having established an office in the Kingdom in 2023, and follows the signing of a Memorandum of Understanding between the companies in 2022 to collaborate on quantum computing capabilities and applications in the energy sector. In 2023, Aramco’s Wa’ed Ventures also participated in Pasqal’s Series B fundraising round.
About Aramco
Aramco is a global integrated energy and chemicals company. We are driven by our core belief that energy is opportunity. From producing approximately one in every eight barrels of the world’s oil supply to developing new energy technologies, our global team is dedicated to creating impact in all that we do. We focus on making our resources more dependable, more sustainable and more useful. This helps promote stability and long-term growth around the world. www.aramco.com
About PASQAL
Pasqal is a leading Quantum Computing company that builds quantum processors from ordered neutral atoms in 2D and 3D arrays to bring a practical quantum advantage to its customers and address real-world problems. Pasqal was founded in 2019, out of the Institut d’Optique, by Georges-Olivier Reymond, Christophe Jurczak, Professor Dr. Alain Aspect – Nobel Prize Laureate Physics, 2022, Dr. Antoine Browaeys and Dr. Thierry Lahaye. Pasqal has secured more than €140 million in financing to date. To learn more about Pasqal, visit www.pasqal.com.
Disclaimer
The press release contains forward-looking statements. All statements other than statements relating to historical or current facts included in the press release are forward-looking statements. Forward-looking statements give the Company’s current expectations and projections relating to its capital expenditures and investments, major projects, upstream and downstream performance, including relative to peers. These statements may include, without limitation, any statements preceded by, followed by or including words such as “target,” “believe,” “expect,” “aim,” “intend,” “may,” “anticipate,” “estimate,” “plan,” “project,” “can have,” “likely,” “should,” “could,” and other words and terms of similar meaning or the negative thereof. Such forward-looking statements involve known and unknown risks, uncertainties and other important factors beyond the Company’s control that could cause the Company’s actual results, performance or achievements to be materially different from the expected results, performance, or achievements expressed or implied by such forward-looking statements, including the following factors: global supply, demand and price fluctuations of oil, gas and petrochemicals; global economic conditions; competition in the industries in which Saudi Aramco operates; climate change concerns, weather conditions and related impacts on the global demand for hydrocarbons and hydrocarbon-based products; risks related to Saudi Aramco’s ability to successfully meet its ESG targets, including its failure to fully meet its GHG emissions reduction targets by 2050; conditions affecting the transportation of products; operational risk and hazards common in the oil and gas, refining and petrochemicals industries; the cyclical nature of the oil and gas, refining and petrochemicals industries; political and social instability and unrest and actual or potential armed conflicts in the MENA region and other areas; natural disasters and public health pandemics or epidemics; the management of Saudi Aramco’s growth; the management of the Company’s subsidiaries, joint operations, joint ventures, associates and entities in which it holds a minority interest; Saudi Aramco’s exposure to inflation, interest rate risk and foreign exchange risk; risks related to operating in a regulated industry and changes to oil, gas, environmental or other regulations that impact the industries in which Saudi Aramco operates; legal proceedings, international trade matters, and other disputes or agreements; and other risks and uncertainties that could cause actual results to differ from the forward-looking statements in this press release, as set forth in the Company’s latest periodic reports filed with the Saudi Stock Exchange. For additional information on the potential risks and uncertainties that could cause actual results to differ from the results predicted please see the Company’s latest periodic reports filed with the Saudi Stock Exchange. Such forward-looking statements are based on numerous assumptions regarding the Company’s present and future business strategies and the environment in which it will operate in the future. The information contained in the press release, including but not limited to forward-looking statements, applies only as of the date of this press release and is not intended to give any assurances as to future results. The Company expressly disclaims any obligation or undertaking to disseminate any updates or revisions to the press release, including any financial data or forward-looking statements, whether as a result of new information, future events or otherwise, unless required by applicable law or regulation. No person should construe the press release as financial, tax or investment advice. Undue reliance should not be placed on the forward-looking statements.
Aramco Contact Information:
@aramco
View original content:https://www.prnewswire.co.uk/news-releases/aramco-signs-agreement-with-pasqal-to-deploy-first-quantum-computer-in-the-kingdom-of-saudi-arabia-302150019.html
-

 Artificial Intelligence7 days ago
Artificial Intelligence7 days agoAdvanced HPC Server Platforms by MiTAC and TYAN Spotlighted at ISC High Performance 2024
-

 Uncategorized6 days ago
Uncategorized6 days agoArtificial intelligence tool detects sex-related differences in brain structure
-

 Artificial Intelligence4 days ago
Artificial Intelligence4 days agoJapan Data Center Market Investment to Reach $14.48 Billion by 2028 – Watch Out Exclusive Insight on Japan & Hong Kong Data Center Market – Arizton
-

 Uncategorized5 days ago
Uncategorized5 days agoGoogle to roll out AI-generated summaries at top of search engine
-

 Artificial Intelligence4 days ago
Artificial Intelligence4 days agoStrava Unveils New Chapter of Accelerated Product Development at Brand’s Flagship Event
-

 Artificial Intelligence4 days ago
Artificial Intelligence4 days agoData Center Colocation Market Worth USD 58.4 Billion to 2031 – Exclusive Report by InsightAce Analytic Pvt. Ltd.
-

 Uncategorized3 days ago
Uncategorized3 days agoCoca-Cola: The future is ‘AI meets human ingenuity’
-

 Uncategorized4 days ago
Uncategorized4 days agoSoftBank looks at ‘softening’ angry customer calls with AI























